The First Law of Thermodynamics States That
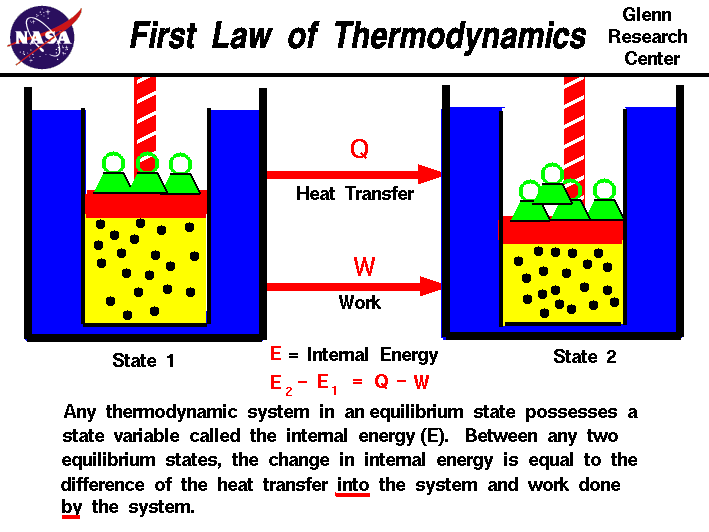
What is the First Law of Thermodynamics?
The first law of thermodynamics is a fundamental principle of physics that states that the total energy of a closed system is constant. This means that energy cannot be created or destroyed, only transformed from one form to another. The first law is also known as the law of conservation of energy.
How Does the First Law of Thermodynamics Apply to Everyday Life?
The first law of thermodynamics applies to everyday life in many ways. For example, when you turn on a light bulb, electrical energy is converted into light energy. When you drive a car, chemical energy from the gasoline is converted into kinetic energy to move the car. When you eat food, chemical energy is converted into thermal energy to keep your body warm.
What is the Difference Between the First Law and the Second Law of Thermodynamics?
The first law of thermodynamics states that energy cannot be created or destroyed, only transformed from one form to another. The second law of thermodynamics states that the total entropy of a closed system always increases over time. Entropy is a measure of disorder or randomness in a system.
Expanding the Keyword: Law of Conservation of Energy
The law of conservation of energy is a fundamental principle of physics that states that the total energy of a closed system is constant. This means that energy cannot be created or destroyed, only transformed from one form to another. The law of conservation of energy is also known as the first law of thermodynamics.
Advantages of the First Law of Thermodynamics
- The first law of thermodynamics is a fundamental principle of physics that helps us understand the relationships between different forms of energy.
- The law of conservation of energy is a powerful tool for scientists and engineers who work with energy systems.
- The first law of thermodynamics provides a basis for the development of many important technologies, such as engines, refrigeration systems, and power plants.
Disadvantages of the First Law of Thermodynamics
- The first law of thermodynamics does not provide any information about the direction or efficiency of energy transformations.
- The law of conservation of energy does not take into account the fact that some energy may be lost as heat or other forms of energy that are not useful for doing work.
- The first law of thermodynamics assumes that energy is a continuous, homogenous quantity, which may not always be the case in real-world systems.
Conclusion
The first law of thermodynamics is a fundamental principle of physics that states that energy cannot be created or destroyed, only transformed from one form to another. This law is a powerful tool for scientists and engineers who work with energy systems, and provides a basis for the development of many important technologies. However, the first law does have its limitations, and does not provide any information about the direction or efficiency of energy transformations.
FAQs
Q: What is thermodynamics?
A: Thermodynamics is a branch of physics that deals with the relationships between heat and other forms of energy.
Q: What is the law of conservation of energy?
A: The law of conservation of energy is a fundamental principle of physics that states that the total energy of a closed system is constant. This means that energy cannot be created or destroyed, only transformed from one form to another.
Q: How does the first law of thermodynamics apply to everyday life?
A: The first law of thermodynamics applies to everyday life in many ways. For example, when you turn on a light bulb, electrical energy is converted into light energy. When you drive a car, chemical energy from the gasoline is converted into kinetic energy to move the car. When you eat food, chemical energy is converted into thermal energy to keep your body warm.
Q: What is entropy?
A: Entropy is a measure of disorder or randomness in a system. The second law of thermodynamics states that the total entropy of a closed system always increases over time.
