The Law of Conservation of Mass States
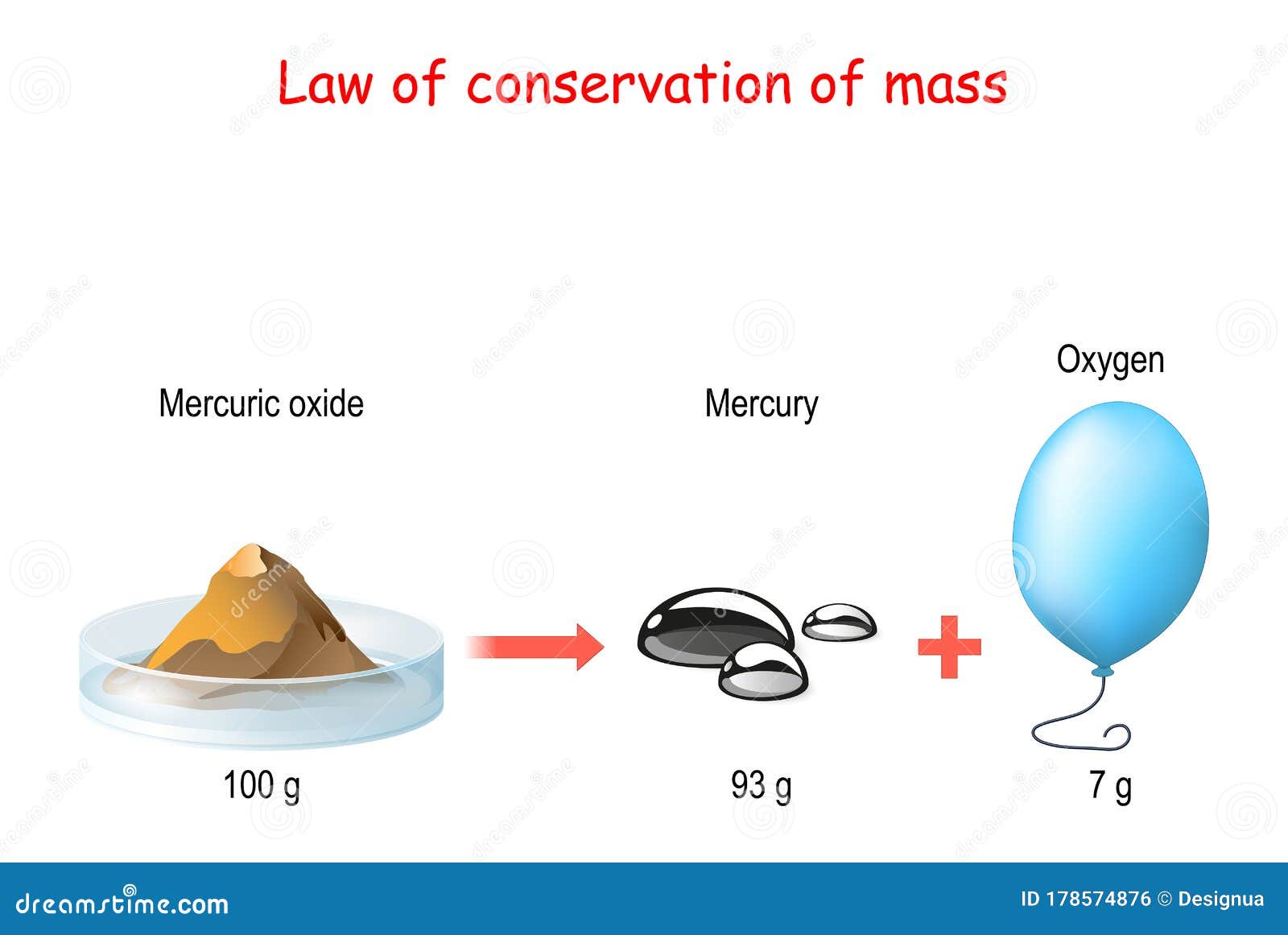
Explanation of the Law of Conservation of Mass
The law of conservation of mass is a fundamental principle in chemistry. It states that in a closed system, the total mass of the system remains constant over time, regardless of any physical or chemical changes that may occur within the system. This means that the mass of the reactants in a chemical reaction must be equal to the mass of the products.
Why is the Law of Conservation of Mass Important?
The law of conservation of mass is important because it allows scientists and engineers to predict the outcomes of chemical reactions and other processes with a high degree of accuracy. By understanding the conservation of mass, scientists can design chemical reactions and industrial processes that are efficient and environmentally friendly.
Examples of the Law of Conservation of Mass in Action
One example of the law of conservation of mass in action is the combustion of gasoline in a car engine. When gasoline is burned, it reacts with oxygen to produce carbon dioxide and water vapor. The total mass of the reactants (gasoline and oxygen) is equal to the total mass of the products (carbon dioxide and water vapor), demonstrating the law of conservation of mass.
Advantages and Disadvantages of the Law of Conservation of Mass
The law of conservation of mass has several advantages and disadvantages:
Advantages:
- It is a fundamental principle of chemistry and physics, allowing scientists to make accurate predictions about the behavior of matter.
- It is a universal law, applicable to all chemical reactions and physical processes.
- It provides a basis for the design of efficient and environmentally friendly industrial processes.
Disadvantages:
- It assumes that there are no external factors affecting the system, which is not always the case.
- It does not take into account the possibility of mass loss due to evaporation or other physical processes.
- It cannot be directly observed, only indirectly inferred from the outcomes of chemical reactions and physical processes.
Conclusion
The law of conservation of mass is a fundamental principle of chemistry and physics. It states that matter cannot be created or destroyed, only transformed from one form to another. This principle allows scientists and engineers to design efficient and environmentally friendly industrial processes and make accurate predictions about the behavior of matter.
FAQ:
1. What is the law of conservation of mass?
The law of conservation of mass states that matter cannot be created or destroyed, only transformed from one form to another.
2. Who first proposed the law of conservation of mass?
The law of conservation of mass was first proposed by Antoine Lavoisier in 1789.
3. Why is the law of conservation of mass important?
The law of conservation of mass is important because it allows scientists and engineers to predict the outcomes of chemical reactions and other processes with a high degree of accuracy.
4. What are the advantages and disadvantages of the law of conservation of mass?
The advantages of the law of conservation of mass include its fundamental nature, universality, and applicability to the design of efficient and environmentally friendly industrial processes. The disadvantages include its assumption of no external factors affecting the system, its inability to account for mass loss due to physical processes, and its indirect inference from the outcomes of chemical reactions and physical processes.
